-
The University
- Welcome
- Who we are
- Media & PR
- Studying
-
Research
- Profile
- Infrastructure
- Cooperations
- Services
-
Career
- Med Uni Graz as an Employer
- Educational Opportunities
- Work Environment
- Job openings
-
Diagnostics
- Patients
- Referring physicians
-
Health Topics
- Health Infrastructure
Alternative Biomodels and Preclinical Imaging
Our goals are to establish and use appropriate human and animal cell culture models and to integrate preclinical imaging into ongoing projects. This enables the development of methods to reduce the number of animals used in tests while increasing the amount of information obtained.
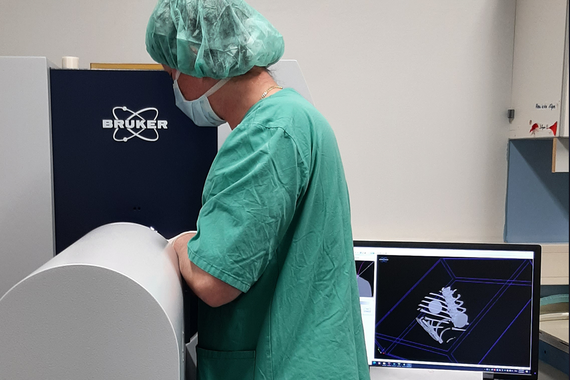
Preclinical imaging
In vivo literally means "in life." In vivo imaging techniques allow us to observe natural processes and diseases in living animals non-invasively and to monitor them over a longer period of time. Using the most modern techniques, we are able to obtain images on a wide variety of levels from full-body imaging to subcellular structures. The following procedures are available:
- Micro-ultrasound: A wide variety of physiological or anatomical structures are visualized as a high-resolution 2D or 3D image using high-frequency sound waves.
- Micro-computed tomography: The different permeability of X-rays of tissues and materials are visualized on 3 levels.
- Fluorescence-based optical imaging: Molecules are detected that absorb the light of a certain wavelength and emit another wavelength (new equipment being purchased).
The ABPCI CF is also responsible for the Biological Irradiator RS2000 (RadSource) and two S2 fields of work.
The ABPCI CF systems can be used for different problems (see applications and technical details regarding the respective systems). The ABPCI CF team would be happy to answer your individual questions on project planning and project implementation.
The University of Graz also has a preclinical magnetic resonance scanner.
Micro-CT
Bruker's SkyScan 1276 High Resolution In-Vivo Micro-CT
Micro-computed tomography is a non-invasive radiological cross-sectional imaging method with very high resolution that can be used for pathological and physiological analysis as well as for material examinations.
An anesthesia unit that can be inserted into the device is available for in vivo testing of small rodents. Thanks to developments in contrast media, it has become possible to depict bones as well as soft parts.
The facility has a high-performance workstation (Dual Xeon with 128 GB storage) for data analysis and data postprocessing in 2D and 3D that uses Materialise MIMICS 22.0. und CTAn (Bruker) software.
In order to deliver a satisfactory result, it is imperative to have a discussion at the start that clarifies the concrete problem explored in the project, opportunities for analysis and the estimated time and cost expenditure. Accordingly, our team will establish and conduct the examinations and analysis.
Applications:
- Quantification of adipose tissue
- Lung examinations
- Soft tissue examinations with contrast media
- Bone structure analysis, bone density tests and implant examinations
- Material examinations
Equipment specifications
In vivo, ex vivo, in vitro
- Cooled CCD X-ray detector
- Detector pixels 4032 x 2688
- Max. FOV 80 x 300 mm
- 20–100 kV
- 2.8 μm smallest pixel (ex vivo)
- 5-6 μm Details with more than 10% contrast
Micro-ultrasound
FUJIFILM VisualSonics' Vevo 3100
The user-friendly Vevo 3100 is the world's first preclinical ultrasound system operated with touchscreens. The only system currently available in Austria is at the ABPCI CF. All four system transducers can be used on different organisms in all possible modes.
Application examples:
- Oncology trials
- Image-guided injection
- Cardiovascular blood flow assessments
- Liver studies
- Organ screening
Equipment specifications:
- Up to 30µm resolution
- Penetration depth up to 30 mm according to the SOP of the Vevo3100 ultrasound system
- Vevo LAB software
- VevoStrain software package
- VevoVasc software package
- Non-invasive testing in real time
- The most accurate cardiovascular assessments
- Physiological monitoring system

CellBank
The CellBank offers a broad palette of services and competent technical support for cell and tissue cultures.
The ISO certification of the core facility guarantees comprehensive quality control of cell lines and a controlled standardized storage system. Cell lines are established and characterized in close collaboration with the Comprehensive Cancer Center at the Medical University of Graz.
Med Uni Graz cell lines
In vitro experiments in different cell systems can reduce and replace animal testing. In any case, in vitro testing provides the basis for animal testing in line with the 3R principle. CellBank Graz supplies Med Uni Graz researchers with human and animal cell lines that comply with a high quality standard.
The list of all cell lines available to Med Uni Graz researchers is available upon request.
Cell lines are also established as required and characterized in detail.
Non-tumorigenic cell lines:
- Human juvenile fibroblasts
- Human adult dermal fibroblasts
- Human juvenile keratinocytes
- MUG Lucifer iHDF immortalized human dermal fibroblasts
- Human juvenile melanocytes
- Human adult dermal melanocytes
Chordoma cell lines:
- MUG-Chor1: Human sacral chordoma
- MUG CC1: Human clival chordoma
Melanoma cell lines:
MUG Mel 3: Human BRAF melanoma from a pregnant patient
Sarcoma cell lines:
MUG CiDus: Human CIC-DUX 4 sarcoma
MUG EMCS: Human extraskeletal mesenchymal chondrosarcoma
MUG Lucifer: Human clear cell sarcoma
MUG Lucifer metastasis: Human clear cell sarcoma lung metastasis
Cholangiocarcinoma cell lines:
MUG CCArly 1: Human cholangiocarcinoma/Klatskin tumor
Quality standards
Mycoplasma detection: The core facility employs one of the most sensitive methods, polymerase chain reaction (PCR), for regular quality control of cell cultures.
Identification of human cell lines: Short Tandem Repeat (STR) analysis with a PowerPlex® 16 system is used for human cell line authentication. The service includes analysis and/or extraction of genomic DNA and comparison of the DNA profile with cell databases.
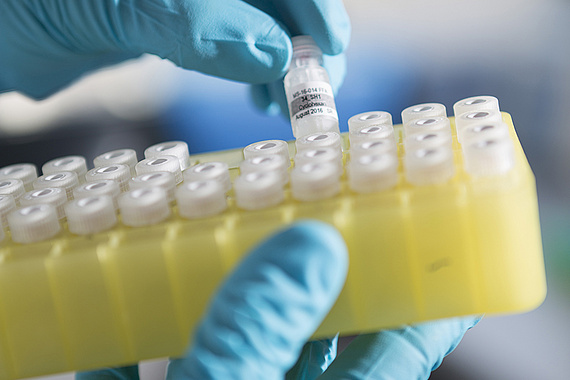
Cell Culture Facility
The Cell Culture Facility, whose human section is located in the Center for Medical Research (ZMF) and human/animal cell culture in Biomedical Research (BMF), supports research by providing cell lines and media, expendable materials and expertise. The facility supplies researchers with 3D spheriod cultures as well as co-culture systems and offers cell culture-based assays such as proliferation assays, viability assays, migration assays and invasion assays. The Cell Culture Facility also makes available a cytostatic cabinet and its existing infrastructure. Our equipment includes:
- ZMF S2 lab
- ZMF cytostatics S2 lab
- BMF S2 lab
- Clariostar photometer
- xCELLigence real-time system
FBS-free cell culture—human platelet lysate (hPl)
Animal-free cell culture models are mandatory for translational research and clinical application of cell culture products. Human platelet lysate (hPl) may be substituted for previously used fetal bovine serum (FBS) in different fields of research, above all for the expansion of mesenchymal stromal cells and in other areas of regenerative medicine. The Department of Blood Group Serology and Transfusion Medicine provides pooled human platelet lysate for research. It is produced from fresh platelet concentrate in ISO 9001:2015 certified laboratories. The human platelet lysate "O platelets in AB plasma" is highly standardized and the aliquots are delivered with a comprehensive quality certificate. GMP-grade O/AB hPl is also available by special request.
Contact: Claudia Maria Bernecker
Isolation and establishment of extracellular vesicles
Extracellular vesicles (EVs) are nanometer-size particles (30 nm to 1000 nm) that can be released by cells and are no longer capable of reproduction. These particles have a lipid bilayer membrane and serve as transporters for various biomolecules. These biomolecules assist in communication, which is why they play an increasingly important role in medical research. In the future, they will be used as biomarkers for a variety of diseases as well as in therapy of diseases by packing substances and transporting them to specific target cells.
The ABPCI core facility has specialized in protocols for isolating and characterizing extracellular vesicles from cell culture supernatants.
Isolation of EVs
EVs can be detected in nearly all body fluids. The ABPCI core facility has specialized in the isolation of EVs from blood plasma and also cell culture supernatants. Depending on the area of application, a choice can be made between ultracentrifugation (UC) and size-exclusion chromatography (SEC).
In 2018 the International Society for Extracellular Vesicles issued a recommendation on how EVs should be isolated and characterized in order to guarantee reproducible and reliable data. When establishing protocols and planning testing, the ABPCI core facility always makes sure to incorporate these recommendations into their work. Link to recommendation
Ultracentrifugation (UC)
Size-exclusion chromatography (SEC)
SEC is becoming more and more popular in the community since this method promises good yields and very native EVs. In addition, isolation proceeds relatively quickly and can also be automated in further steps.
Currently EVs are isolated from platelet-free plasma (PFP) with Sepharose SEC columns that can be precipitated beforehand with polyethylene glycol (PEG).
Flow assisted cell sorting (FACS)
In addition to electron microscopy, FACS is presumably the most important method for investigating EVs. The great advantage here is that individual EVs can be investigated one after another for several markers at once. Due to the small size of the particles, special methods and settings should be used that are very different from "normal" FACS measurements.
Nano Tracking Analysis (NTA)
NTA is used to estimate the size distribution (30nm to 1000nm) and particle concentration of EVs in samples. The particles are excited by a laser. The Brownian motion of their particles is then filmed by a microscope. This footage is evaluated by a software program and the size as well as the concentration of the measured particles is calculated.
Western Blot (WB)
WB is mainly used to characterize the isolated EVs. The purity criterion is tetraspanin markers (CD9, CD63 and CD81), which must be detected. Depending on what biomaterial is used, there are negative markers that ideally cannot or can hardly be detected in samples. In the case of blood, it is the marker (e.g., apolipoprotein A1) for lipoprotein particles that often contaminates EV samples.
3D cell cultures and co-cultures
The cultivation of cells in a microstructured three-dimensional cell culture with different cell types reflects the in vivo situation and is optimal for testing chemotherapeutics.
The ABPCI core facility provides 3D tumor models in different matrices and scaffolds as well as embedding and staining protocols.